The life of a pterosaur cannot be easy. Most occur in places where there is always a larger predator; even the giants Quetzalcoatlus and Hatzegopterus may have co-occurred with other predators that would have seen them as food. From hatching to growth and eventually the necessary act of breeding – to pass one one’s genetic legacy, a primal, incomprehensible need – the pterosaur marches inexorably to death, changing the world as it goes.

Dr. Toni Bürgin of the Naturmuseum St. Gallen and Hr. Urs Oberli, of Switzerland, together holding the holotype of Banguela oberlii. Very delicately, as the specimen is in two pieces. It is easy to see how actually large this pterosaur’s jaw is, and it would have rivaled the sizes of many other Santana pterosaurs.
This is much the same way that a pterosaur fossils finds its way from recognition to publication. Hebert Campos and I are not the first to work with this specimen, and likely not the last. Two previous works lie before us. Veldmeijer et al. (2005) initially described the specimen, and Humphries et al. (2011) used the specimen in a flume to test its effectiveness as a skimming tool (answer: it’s not very efficient at skimming). Seven researchers total have looked at the specimen and found it interesting enough to deal with in a formal publication. My involvement with the specimen adds three more names, one of whom is not an author on the paper. Given the number of people working on the specimen, one might think we’d run out of things to say, but that turned out not to be the case.
My involvement began without even knowing of the specimen’s existence, in 2011. Along with Hebert and Nick Gardner, I was working on Thalassodromeus sethi, quite possibly the most underrated pterosaur I know of. After trawling for all work regarding this taxon, we re-discovered the work on “SAO 251093.” Its peculiarity struck us, and we delved deeper. We looked into the initial arguments around the specimen, and began comparing it back and forth across pterosaurs, to see if those peculiarities might be resolved. See, Veldmeijer et al. had considered either dsungaripterids or Thalassodromeus as potential containers for “SAO 251093” and had listed a few features here and there. Looking into Dsunagripteridae, however, suggested a few more. We had access to extensive data on Thalassodromeus, so that was incredibly useful as well.
However, Thalassodromeus data is being held pretty close to our lockboxes, so we couldn’t say much. I started writing some stuff up, getting an idea of what it seemed the specimen was telling us, and passed this back and forth across Hebert and Nick. Eventually, I got a good direction (it seemed to be a new species) and we started looking into formalizing the arguments. There’d be a paper.
It’s 2012 now, and I’m only just now getting a MS together. Hebert and I are working on it, so we’ll be the authors. Nick is functioning as project lead, but this was our contribution. But it would be a poor thing of me to say that Nick didn’t contribute, as I said before, and this paper would have three authors were it not for his reluctance. One can say that advisors and such shouldn’t be on papers, and that’s true; but when you actively contribute to the work, as Nick did – and not a little at that – as well as discussions on experimental work (Nick also helped with the phylogenetic analysis in the paper) authorship should be guaranteed. C’est la vie.
The first thing we started to address is the problem of the descriptions in pterosaurs. For the most part, pterosaur studies are about three things: skulls, vertebrae, and pectoral girdle with limb bones. There are other parts, but they tend not to get attention. Analyses regard the pelvis and jaw of pterosaurs, but rarely in sufficient detail. So we had to do a lot of foot work through the pterosaur literature and delve into the personal photographs of pterosaur jaws we’d been developing. I tasked myself with handling the phylogenetic analyses aspects, initially to scan for data and eventually to making a list: Characters that were in discussions but not in phylogenetic analyses of pterosaurs. From 2003 to 2013, there have been numerous phylogenetic analysis, but of these few characters of the mandible (that are not also of the teeth in general) have been included. Briand Andres’ work has been great in helping fix this as well, but not phylogenetic analysis0 This despite being an important element, often of diagnostic value. The same is true of the pelvis, and this gets partially resolved in 2013 by Darren Naish and his colleagues when they discovered the specimen that would become Vectidraco daisymorrisae, but prior to that this isn’t the case.

Results of our phylogenetic analysis. The figure in the paper is flawed by omission of Vectidraco daisymorrisae, but here it is included properly. Some analyses differ in whether Thalassodromeus + Tupuxuara are close to Tapejaridae, and instead are closer to azhdarchids. We will have to add the characters to a broader analysis and see what effect that work has on this placement, as well as Banguela oberlii’s. Numbers reflect nodes, and underscored numbers represent bootstrap values for clades: Thalassodromidae has lower values than Banguela + Dsungaripteridae.
Filling holes in the phylogenetic analyses meant that I had to cross-compare a lot of them, and so I have a nice huge document working on that (it’s still not done yet, I’ve been lazy). But I had a huge chunk of data and it turned out that there are diagnostic features in the jaws that haven’t been touched on. I’ll get to this at some later time. But the next problem that came up was qualifying what we saw that hadn’t been discussed before. Among pterosaur jaws, only a few works stand out: Peter Wellnhofer and André Veldmeijer both have addressed anhanguerid jaws (Anhanguera and Coloborhynchus, respectively), but mostly dealing with teeth; and Chris Bennett on Pteranodon in a comprehensive manner.
You really have to hand it to the latter work: when it comes to describing the jaw, Chris Bennett’s work is pretty damn good. But the problem lies in the fact that of these works, little comparative work has been done on pterosaur jaws. As a jaw guy, this astounds me. When it comes down to it, the Banguela paper is actually an introduction to the morphology of a pterosaur jaw. We approached the text with an understanding to put some of the new work Casey Holliday and others have been putting into in sauropsidans in general into terms of pterosaurs, with my own added twist. (It didn’t work as well as I wanted, but that I think has more to do with the constraints on space we imposed on the paper.) We also did something that no one had done before for pterosaurs, and that was begin discussing the mandibular symphysis and a complex of multiple features relating to it.
Where the two halves of the mandible comes together, a symphysis (a joint between bones) occurs. In the most primitive pterosaurs, the two halves of the mandible have an open suture, and the two are likely somewhat mobile. This is probably the case in Anurognathidae. But in most pterosaurs the symphysis is fused. This symphysis varies in size from pterosaur to pterosaur, and from dimorphodontids to rhamphorhynchids it’s confined to a single scooped out platform. Almost all animals with fused jaws are similar to this, but a few have gone a step further. There’s the shelf (the mandibular symphysis itself), and then there’s two.
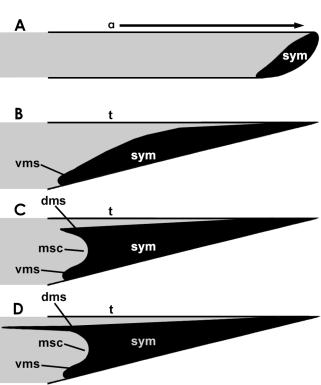
A figure excluded from the paper due to space and scope. schematic representation of the different mandibular symphyseal morphologies in pterosaurs. A represent the basal pterosaur condition, and resembles the anurognathid condition; B represents the “classic” rhamphorhynchoid morphology, as in Eudimorphodon and Rhamphorhynchus; C represents an hypothetical intermediate between B and D, and is also known in dsungaripterids; and D represents virtually all pterodactyloid pterosaurs in which the upper shelf (dms) is distinctly longer than the ventral one (vms). Both shelves create a “mandibular cavity,” here termed a mandibular symphyseal cavity (msc). “a” = alveolar margin, “t” = tomial margin.
After splitting from rhamphorhynchids, other pterosaurs form a group called Pterodactyloidea. Pterodactylus antiquus is the essential pterodactyloid, and it has two symphyseal shelves, one above the other, and with a space between called a “mandibular cavity.” The shape, relationship, and formation of these shelves have hardly ever been touched on. And as it turned out, these factors are diagnostic in some pterosaurs. We knew going into this project might reveal some interesting things, but this was a surprise. Now, other groups of animals have similar adaptations, principal among them turtles; but even within them this feature has received scant attention. I might suggest that the upper shelf develops as it seems it does in turtles by growth of the medial ridges of the jaw, converging together and forming a shelf; a similar thing may have happened to oviraptorosaurs. But this bears testing.
As I said, a large portion of the paper was about qualifying a few of these characters. It started at seven. It eventually grew to nine. I now have 14. That’s fourteen mandibular characters that hadn’t been qualified, among 18 I added to the analysis in the paper. Discussing these features formed the major section of the paper. In addition, I decided to work on the comparative morphology of mandibles a little and provided a justification for referral to Pterosauria. Normally, people may just declare it outright, but I had reason to doubt. I was mindful of UCMP 143274, the “Lance parrot” I discuss here.
One of the criticisms that jaw produces is the problem of identifying whether it is a bird or not, or whether it may be something else. We have clues, though, in comparative anatomy. And those clues are visible in NMSG SAO 251093. So we addressed that. It’s a minor section, but I think a valuable one, if at least pointing out the importance of qualifying your taxonomic identification beyond listing autapomorphies. We did that, but too; not merely just.
We also realized we probably had a dsungaripterid. And there were a lot of things to say about that. Well, it’s the youngest one, potentially the biggest one, and it’s from South America. What might the upturned jaw mean among Santana pterosaurs? Paleobiology, biogeography, grand designs for a paper about a little fragment of jaw. The manuscript I ended up writing by the end of 2012 was a monstrous 30+ paper. But we submitted it to PLoS ONE anyway. And deservedly, the paper was rejected, but not before some of the problems of peer-review and the editorial gateway came through.
Only one of our reviewers was polite, and in this was very careful to list out the difficulties. The other, however, was far more problematic. It is hard to read the response that reviewer gave and think good of the writer. But we saw the first reviewer’s work, though arguing for rejection (and refusal to actually review the paper in full in its present form) as taking the correct stance. Sometimes you need a review like that. It would be a while before we went back to the MS again. This time, though, when I thought I got it into some shape, I sent it to some friends for pre-review. They, too, could not review it in its current form. The reasons given though, were very plain: There’s a lot of crap in there we didn’t need; whole swaths of it should go. Cut the paper to less than 10 pages. From 30.
>So I took a hatchet to it. Out went the biostratigraphy, the paleobiology (only a fragment of it remained at the end, a small bit that ended up getting the axe through a later round of review). Retooled. Down to 15. Further. 14. Then the hardest part: rewrite it, to remove the passive voice, weasel words, the words that are nothing but glam. I have some difficulties with this still, and am far better at first person narrative than third-person “science speak.” But I tried. 11. I didn’t think I could get it down further. I cleaned up the text more, dropped a figure, and then … submission. Not to PLoS ONE. Problematically, our editor had indicated that we would not be accepted if we resubmitted merely because neither Hebert or I were actively enrolled in an academic institution or degreed. I wanted to aim for OA, but our choice was opened for us with the Rio Pterosaur Symposium, a specialty of pterosaur workers who’d be looking at the papers.
 Off it went. We got a surprising turnaround on the reviews, and neither rejected; but wanted major revisions. These I did in about two days, including a request for the phylogenetic analysis that’s in the paper. It was submitted without one, and its addition increased the page count by one. But it helped affirm an hypothesis of ours, the dsungaripterid nature of Banguela oberlii. It was around this time that we realized that it was a good idea to name the species, not merely do so as a pro forma measure of specimen description. Sure, it’s a distinct specimen, but is it a new species? We could, with more confidence, say that it was not Thalassodromeus sethi. The paper’s purpose was now simple: introduce the specimen and provide its historical context; introduce the problem of context and morphology, and suggest solutions; work on resolving the problem, performing comparative and phylogenetic analyses; synthesize the results, which affirm the hypothesis of both dsungaripterid identity and the presence of a new taxon; and name it.
Off it went. We got a surprising turnaround on the reviews, and neither rejected; but wanted major revisions. These I did in about two days, including a request for the phylogenetic analysis that’s in the paper. It was submitted without one, and its addition increased the page count by one. But it helped affirm an hypothesis of ours, the dsungaripterid nature of Banguela oberlii. It was around this time that we realized that it was a good idea to name the species, not merely do so as a pro forma measure of specimen description. Sure, it’s a distinct specimen, but is it a new species? We could, with more confidence, say that it was not Thalassodromeus sethi. The paper’s purpose was now simple: introduce the specimen and provide its historical context; introduce the problem of context and morphology, and suggest solutions; work on resolving the problem, performing comparative and phylogenetic analyses; synthesize the results, which affirm the hypothesis of both dsungaripterid identity and the presence of a new taxon; and name it.
This final element of the process is an important one for me. Naming Banguela oberlii is one of the last things the paper does. It is my belief that systematic paleontology is a result of analysis, not a preclusion of it. When you present a paper, the process is always to introduce the principles, relate the problem, resolve the problem, then conclude. For us, this would be Introduction, more Discussion, Methods and Analysis, then Results.
Too commonly, taxonomy is introduced before the analysis is. Mostly, this is because you want to talk about the taxa you mention without cluttering things with specimen numbers. Yes, it is cleaner, but it isn’t necessarily better. This may be most strongly due to the illusion the taxonomy-first approach gives, of informing the reader or analyst that the taxonomy is more important, the analysis an afterthought. Similar works are produced without the understanding that the analysis undergirds everything else, and should be more important. Yes, you have more paper to wade through before you get to the name, but I think that helps teach the reader that we’re talking about a specimen, then we talk about a species.
Early drafts of the paper refused to name the taxon until the Systematic Paleontology section. But I changed this with its inclusion in the abstract and as one of the keywords. You’ll know the paper names a new taxon, but you’re gonna read through why first. My apologies.
Banguela comes to us via my own evolution as a writer, and as an academic. Getting the work that would become this paper published was a labor, one in which I briefly considered abandoning. After the initial rejection, I put it aside to set up some other projects (including, coincidentally, preparations for trying to get better info on the “Lance parrot,” UCMP 143274) and finally came back to it. Beat the thing into submission, literally.
I’m not the worse for wear for the rocky review process, but I am glad that not only was the work able to get through, the help of people whose advice I valued greatly improved it. I fought for this paper. As I was reminded after the rejection, it was good for me to get it. Maybe not as bad as it was, but nonetheless. This is because the process of revision and understanding of the problems your peers may have with your data do represent valid concerns. When it came to the reviews of the latest round, the paper substantially leaner and focused, I was able to look through the arguments far more rationally.
That doesn’t mean we have to agree with our reviewers, however. We had the right to stand up for some of our decisions (where the naming of the species goes, for example) and because only one reviewer suggested such a change we felt we could keep it that way. Stephen King – probably the last person you’d hear me cite – says of writing that when he passes work to a reviewer, if only one person mentions a problem, he will ignore that comment – he’s the writer, after all, and overrules any one comment; but if two say the same thing, he notices; and the more have the same issue, the more real the problem becomes. With reviews, it is much the same way.
You have Stephen King to thank for the way the paper is presented, for good or for ill, and once again, my apologies. But think of it this way. Our hard work presents one way a paper may be given, a perspective, of data first. I like this over virtually any other method, and part of me righteously thinks this is the best way. If I have to sneak taxonomy into a paper, it will be at the back end, but I’m not going to hide it in an appendix, either.
Banguela oberlii is something that’s worth reading about, in my opinion. We set about to determine if this jaw was Thalassodromeus sethi, and are confident that it is not. We set about to determine what it actually was, and are reasonably confident it represents a definitive record of a typically Eurasian group in South America; it also enriches the Brazilian pterosaur fauna. It has history. It also tells us that many things going on in the Early Cretaceous were not as simple for pterosaurs as we’ve thought, with Laurasian taxa appearing in Gondwana in the form of Nyctosauridae and Dsungaripteridae, Ornithocheiridae and Lonchodraconidae, etc. But that’s a story for another time.
Headden, J. A. & Campos, H. B. N. 2014. An unusual edentulous pterosaur from the Early Cretaceous Romualdo Formation of Brazil. Historical Biology [Published online ahead of print]: 1-13. doi: 10.1080/08912963.2014.904302
Humphries, S., Bonser, R. H. C., Witton, M. P. & Martill, D. M. 2009. Did pterosaurs feed by skimming? Physical modelling and anatomical evaluation of an unusual feeding method. PLoS Biology 5 (8): e204.
Naish, D., Simpson, M. & Dyke, G. 2013. A new small-bodied azhdarchoid pterosaur from the Lower Cretaceous of England and its implications for pterosaur anatomy, diversity and phylogeny. PLoS ONE 8 (3): e58451.
Veldmeijer, A. J., Signore, M., Meijer, H. J. M. 2005. Description of two pterosaur (Pterodactyloidea) mandibles from the Lower Cretaceous Santana Formation, Brazil. Deinsea 11: 67-86.



“For us, this would be Introduction, more Discussion, Methods and Analysis, then Results.”
I agree with this. We did the same for Neptunidraco: we wanted the reader to follow our arguments and then to conclude with us that the specimen was a new taxon, not the opposite.
Pingback: An Edentulous Dsungaripterid? 10 Facts About Banguela | The Bite Stuff
Pingback: A Look Back at the Bite Stuff, 2014 Edition | The Bite Stuff