 The new small theropod Yi qi was described 29 April, 2015, far too late to be a practical joke for All Fools’ Day (by 4 weeks, precisely). Why would it be? The animal, described by Xu Xing and a number of colleagues, is based on a single specimen of a mostly crappy slab.
The new small theropod Yi qi was described 29 April, 2015, far too late to be a practical joke for All Fools’ Day (by 4 weeks, precisely). Why would it be? The animal, described by Xu Xing and a number of colleagues, is based on a single specimen of a mostly crappy slab.
Peculiar to all other non-mammlian volant vertebrates, a bony spar appears to be present attaching to the wrist and is related to carbonized material distinct from preserved feathery filaments that suggests a membrane around the arm. This is a very exciting, and wholly unexpected conclusion. For a while, we’d assumed that maniraptorans retained a basic, if size-variable, model for their arm. But Scansoriopterygidae, beginning with Scansoriopteryx heilmanni (as Epidendrosaurus ningchengensis), revealed that the typical digital model and limb construction was more variable than thought.

Extended data figure 2 from Xu et al. (2014), depicting various aerial-performing “wings.” A-C depict possible reconstructions of the “wing” in Yi qi. D, a bat; E, a bird; F, a (rhamphorhynchoid) pterosaur; G, a petauristine sciurid (squirrel). Yellow depicts the “styliform element” in those animals that have them. In the mammal, this structure arises variously from a modified pisiform or as a sesamoid, an intramembraneous bone and not an endochondral limb bone.
Living birds typically have a thin membrane supported by a ligament that passes between the shoulder and wrist and forms a propatagium. This membrane and ligament is present in bats as well but is supported by a substantive muscle (the m. patagialis longus), and was discovered, with a bony support at its base called the pteroid, in pterosaurs (with researchers like Bennett and Witton providing a similar muscle analogy for a pteroid ligament. These structures lie on the leading edge of the forelimb, whereas the structure in Yi qi lies behind the wrist. Unlike patagial structures on the leading edge, patagial structures behind the arm are less well described. Birds have one, but it’s small. Peculiarly, a small “brachiopatagium” — a membrane between the body wall towards the back of the arm or elbow — exists in numerous terrestrial mammals (e.g., dogs, cats, rats, etc.) in which the elbow is crooked, but also present and often deemed “large” in some primates, such as sifakas and galagos (Deems et al., 1991).
Gliding mammals are diverse. Not restricted to squirrels, there are various groups of both marsupial and placental mammals with skin flaps that help them in gliding flight, forming brachio/cheiropatagia. In marsupials, sugar gliders (Petauridae, Petaurus), greater gliders (Psuedocheiridae, Petauroides), and phalangers (Phalangeridae, Acrobates) exhibit a condition similar to placental “flying squirrels” in bearing a skin membrane covered in fur on both sides stretching between wrist and ankle, leaving the hands and tail free. The tail in Acrobates is very fluffy, spreading out and resembling the tails of many early birds like Archaeopteryx. In contrast, the “flying lemurs” Galeopterus and Cynocephalus (Cynocephalidae) developed a more extensive membrane that compares to bats in that webbing extends between the digits of hands and feet, extends from the ankle to the tail-tip (uropatagium) and the wrist to the neck (propatagium), providing an extensive flight membrane creating a hexagonal, rather than rectangular, gliding surface. Rodents include four distinct lineages of glider, and all of them appear to have developed their gliding surfaces independently (Storch et al., 1996): the Sciuromorpha produces gliding glirids (Glirulus lissiensis Hugueney & Mein, 1965) and sciurid squirrels (Petauristini or Pteromyini sciurids), whereas the Myomorpha produces gliding anomalures (Anomaluridae, with all but one taxon bearing a flying membrane). Eomyids, producing the volant Eomys quercyi, are castorimorphan rodents related to kangaroo rats (Storch et al., 1996).
Remarkably, several of these lineages developed a calcified cartilage “spar” (termed styliform cartilage) extending through the membrane from the arm and forming a part of the leading edge of the gliding surface. In petauristins, this styliform cartilage has the form of a bony sickle-shaped element that attaches to the wrist between the pisiform and scapholunate bones (for which a joint exists) (Thorington & Darrow, 2000); in all other gliding placentals (including both fossil species identified, the gliding species of Eoymys and Glirulus), this element is a cartilage spar that extends from the olecranon process of the ulna, and is likely not homologous with the petauristin element. Sowing yet more confusion, the term “styliform” is used for the cartilage spar that extends medially from the ankle in bats and supports the trailing edge of the uropatagium. In colugos, while a similar extent of membrane as in bats occurs, the only cartilage support structure is at the elbow (Deems et al., 1991). Such a structure is absent at either elbow or wrist in marsupial gliders. In all these mammals, both the appearance of the skin membrane and the spar that supports it converges across short (within various small, scansorial or arboreal clades) and large (between marsupial and placental) phylogenetic distance, with seeming small phyletic variation (the general morphology of almost all gliders excepting colugos appears small, their archetypal shapes being highly conserved with a large, triangular head, splayed limbs, elongate forelimbs and tail, which is often bushy).
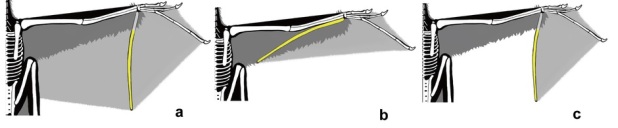
Excerpt from extended figure 2 of Xu et al. (2015), showing variation of wing models for Yi qi. In all cases, a membrane is assumed, as in colugos and bats, along with flying frogs (Rhaceodactylus spp.) and flying geckos (Ptychozoon spp.), to stretch between the fingers.
So it seems with Yi qi. Amongst Scansoriopterygidae, its holotype (STM 31-2, a specimen recovered by a farmer very shortly before being acquired by the STM for preparation and study) is the only specimen of this group for which a skeletal element to support a membrane may be inferred. Xu et al. partially diagnose Yi qi, whose name means “strange wing,” on this feature[n1]. In all other specimens, no such element appears present, even if misidentified (Xu et al., 2015). Furthermore, Xu et al. (2015) report the presence of organic remains they identify as pertaining to dermis comprised of intermittent, scattered and tattered remnants of a filmy black tissue. This substance, clustered around portions of the limbs but also elsewhere on the slab, are claimed to be part of a brachiopatagium, which the styliform bony element would have extended into a functional flight membrane reminiscent of colugos and other placental gliders. The authors, in the main body of the text and in their supplemental discussion of the specimen, are optimistically speculative over the nature of this tissue, but one concern I have about it is that little is done to differentiate it from the organic remains of microbial mats. These mats are composed of the remains of cyanobacteria that, like the skin and liver and eyes of vertebrates, contain melanosomes which may cause confusion with actual remains of soft-tissue of more complex organisms. In many cases, the preservation of more complex tissues is aided by the presence of layers of cyanobacteria within the sediment, as these layers form a firm substrate onto which fossils can deposit in otherwise viscous material such as silt and ash-laden sand. More importantly, the textures of these often take on the properties of adjacent tissues such as feathers, fur, etc., and help the identification of these tissues. However, they may also directly preserve, and this constraint is normally achieved through microscopic and chemical analysis of the tissues. While Xu et al. performed chemical analysis of portions of the STM 31-2 holotype, they did so only for some portions of it, and none of those portions included the membrane. SEM of the material suggests presence of phaeomelanosmes; phaeomelanin is responsible for some reds, yellows, and browns, but is present in varieties of bacterial, algal, and animal life.
Taking Xu et al.’s concerns, it seems problematic to assume the presence of a membrane. As they note, such a structure is not indicated in any other fossils so far found in the Early Cretaceous of China. This includes pterosaurs, for which a skin membrane extending from the forelimb is directly indicated by a variety of fossils around the globe. Indeed, several fossils of pterosaurs show the flight membrane tends not to form a blackened film in this form. This caution is further emphasized by areas of preservation in STM 31-2 in which this film appears to lay in layers separate from that of the filamentous “fuzzage” covering the body. In one area (shown in Xu et al., 2015’s fig. 2 g), this film lies above the distal phalanges of one hand, whilst directly adjacent to the bone and in the layer on which the bone rests feathers are present, consistent with layers of microbia below and above the specimen — but not in the same plane, as might be assumed for pterosaurs or an integument in which the feathers would extend from the skin.

Excerpt from Xu et al. (2015), distal manual digit 1 of STM 31-2, showing joint between md1-1 (left) and mc1 (right, incomplete). Identification of elements as “digit II” refers to Xu et al., refering to anatomical digit 1 as avian homologous digit 2.
So is it skin? And if so, is it a membrane rather than a patch of perhaps denuded or apterial skin? I don’t think so, to either possibility, but the presence of a bony element consistent with a styliform element suggests that some form of stretched membrane may be present. This is the where the convergence of two distinct points of evidence imply a relation between the two; would either have been alone, the conclusion that could be drawn from that point would be weaker. A black film alone doesn’t imply skin membrane, but the styliform element? Two such elements are present in STM 31-2; one lies near the extended left manus, but is incomplete proximally, and the wrist region is not preserved, while the other lies adjacent to the right manus and the manus is nearly complete. Both are curved, and the one element that is “complete” contacts the distal ulna of the right arm. This is where things get tricky, because the element appears to pass beneath the arm:

Excerpt from Xu et al. (2015), preserved right manus of STM 31-2, holotype of Yi qi. right styliform element is not abrupt where it passes below the right ulna, but seems to extend beneath it, and disappear into the slab. Thus it’s continuation and articulation (if any) is problematic.
If the element is a styliform, it’s an odd one. Rather than cylindrical, this element is described as “flattened,” and bears more resemblance to a typical limb bone. However, there doesn’t seem to be a typical limb bone which this may belong to, and while one can suggest the elements come from another animal, the lack of other elements and its consistent pairing left and right and connection to the manus on both sides implies otherwise. We are left, then, with the combined data points: A styliform element lies within a stretched skin membrane, and forms either part of the leading edge (petauristin/pteromyin squirrels), trailing edge (bats), or as an extension of the membrane laterally (colugos, anomalures, eomyids, glirids). Xu et al. seem to prefer the lateral interpretation, with tissues connecting to the hyperelongate third digit of the manus towards the body wall. However, in all mammals in which this element is present with membrane to either side, such a membrane extends from at least elbow to knee, or wrist to ankle, and one wonders if this should be the case in Yi qi.
The novelty of their reconstruction is extremely bizarre, but not unprecedented. Indeed, in his attempt to figure out possible models for bird wing origins, Colin Pennycuick in 1986 proposed a model that included a thin membrane of skin that stretched between the fingers and from the hand to the torso, and it would be from this membrane, he argued, that the quills of feathers would insert. This was an elaboration of the “arboreal leaping” model for bird origins, and has since been argued as relatively untenable. It was, in this model, a means of explaining many terrestrial adaptations in the legs of birds, adaptations that in the terrestrial model were the products of dinosaurian ancestry; the two models were in opposition, not because they were overly simplistic in the ground-up/trees-down dichotomy debate, but because the leaping model assumed that the digits of birds had to have arising as a reptilian, lizard-like tree-climber, and was a continuation of the “thecodont origin of birds” theory that has since shown to have no teeth (pun groaningly intended). We know this model is overly simplistic because birds can jump; but Pennycuick’s model proposed an intermediate in which the splayed fingers coalesced as feathers developed, from a bat-like spread-hand model into a modern-bird model. While this model has proven weak, the recovery of scansoriopterygids recently (Czerkas & Yuan, 2002; Zhang et al., 2002, 2008) suggests some radical revisions during the mosaic evolution of maniraptorans. It’s also remarkably similar to Dougal Dixon’s “harridan,” which was however considered a modified but “true” pterosaur (Dixon, The New Dinosaurs), published in 1988.
At top, I present different perspectives on this material. A bat-like but colugo-squirrel-like feathered membrane would connect to the knee and form a continuous membrane. I find this model more likely than just a torso-wrist connection, as this seems to be the case in virtually all other membrane-winged vertebrates known to date. There is also a bottom-view in which the landing posture resembles that of many gliding mammals in a full vertical tilt. However, in contrast to them, the hindlegs are not splayed, and thus appear more avian in their posture. An illustration accompanying Hone’s commentary in The Guardian includes an illustration (from Dinostar Co. Ltd.) that depicts a splayed hindlimb, but this is an outlier amongst recent depictions. The effectiveness of such a landing was probably just as it needed to be, but whether it could land on a tree-trunk in this manner seems questionable. There is also a reconstruction of a membrane-less, and rather oviraptorosaurian, scansorioptergid. Most, if not in fact including Yi qi as well, scansoriopterygids would have looked like this.
Finally, a less peculiar note.
In Padian’s (2015) commentary on the discovery, the editors to Nature included an illustration that lays out a comparison of the scansoriopterygids, of which there now seem three distinct morphs.

Scansoriopterygids. Attributed to Xing Lida in the caption.
These illustrations bear an uncanny resemblance to my Scansoriopterygidae illustrations:

Scansoriopterygidae, including all referred taxa. Scansoriopteryx heilmanni is often considered synonymous with Epidendrosaurus ningchengensis, both are probably juveniles, and they may in turn be synonymous with Epidexipteryx hui (in which case Scansoriopteryx heilmanni has priority above both other taxa).
In fact, with the exception of some alterations to the skull in A and B, the lifted foot being dropped down, some parts being moved around, and missing elements of various parts filled in or other elements erased (the sternum in Epidexipteryx hui is removed), these skeletal illustrations are mine. I feel like I went through something like this before, but this time the issue is different. This time, my skeletal material, covered on a CC-BY license, which merely asked for my name to accompany the piece regardless of alteration, is being appropriated and the person doing the alteration is being given credit. So … my art is now in Nature, and for that I get nothing.
[n1] Other features of the skull are used to diagnose Yi qi: the shape of the premaxilla, the shape of the teeth in the premaxilla and the presence of a basal oviraptorosaur-like large first premaxillary crown (stressing the convergence and possible similar ancestry between the two groups), the relative size of maxilla and premaxilla is smaller, a smaller external mandibular fenestra, relatively longer forelimbs with a shorter, with a higher deltopectoral crest of the humerus.
Czerkas, S. A. & and Yuan C.-q. 2002. An arboreal maniraptoran from northeast China. in Czerkas, S. J. (ed.) Feathered Dinosaurs and the Origin of Flight. The Dinosaur Museum Journal 1:63-95.
Demes, B., Forchap, E. & Herwig, H. 1991. They seem to glide. Are there aerodynamic effects in leaping prosimian primates? Zeitschrift für Morphologie und Anthropologie 78 (3): 373-85.
Hugueney, M. & Mein, P. 1965. Lagomorphes et Rongeurs du Nèogène de Lissieu (Rhòne) [Rodents and lizards from the Neogene of Lissieu (Rhône)]. Travaux des Laboratoires de Gèologie de la Faculté des Sciences, Lyon 12: 109-123.
Padian, K. 2015. Palaeontology: Dinosaur up in the air. Nature (Online before Print 29 April, 2015) DOI: 10.1038/nature14392
Pennycuick, C. 1986. Mechanical constraints on the evolution of flight. Memoirs of the California Academy of Sciences 8: 83-98.
Storch, G., Engesser, B. & Wüttke, M. 1996. Oldest fossil record of flying in rodents. Nature 379: 439-441. DOI: 10.1038/379439a0
Thorington, R. W., Jr. & Darrow, K. 2000. Anatomy of the squirrel wrist. Bones, ligaments, and muscles. Journal of Morphology 246 (2): 85-102.
Xu X., Zheng X.-t., Sullivan, C., Wang X.-l., Xing L., Wang Y., Zhang X.-m., O’Connor, J. K., Zhang F.-c., Pan Y.-h. 2015. A bizarre Jurassic maniraptoran theropod with preserved evidence of membranous wings. Nature (Online before Print 29 April, 2015) DOI: 10.1038/nature14423
Zhang F.-c., Zhou Z.-h., Xu X. & Wang X.-l. 2002. A juvenile coelurosaurian theropod from China indicates arboreal habits. Naturwissenschaften 89(9):394-398.
Zhang, F.-c., Zhou Z.-g., Xu X., Wang X.-l. & Sullivan, C. 2008. A bizarre Jurassic maniraptoran from China with elongate ribbon-like feathers. Nature 455:1105-1108.



Hey Jaime, have you contacted Nature, or the Authors? I’m sure you have but if not please do to see if you can get some kind of credit/recompense.
I am going to. I have to craft the comparison figure, then I will discuss it.
Good luck! Hope you get some joy. :)
Someone should organise a protest about all the art theft.
Scansoriopterygids, to my knowledge, had more flexible hip-sockets than other dinosaurs. Almost certainly useful for arboreality, but if Yi had a gliding membrane it would almost certainly suggest that it was splaying its legs.
Dixon’s New Dinosaurs had another creature called the flurrit which is more similar to Yi– a gliding theropod with a membrane similar to a colugo.
It even has what appears to be styliform element.
The purported radius is an ulna splinter on both arms. The styliform element is the radius. See pterosaurheresies.wordpress.com for details. This is occam’s razor at its worst.
That isn’t true. The radius and the syliform are clearly separate skeletal elements.
And here lies a major problem with why people won’t take you seriously. You won’t take the evidence and the review of it seriously. Let me me be clear by rephrasing your comment:
“I think that the purported ulna is splintered on both arms, and one of these was identified as a radius. My evidence for this is that I redrew the material to describe two distinct elements on one side of the slab as two splinters of an ulna. I think that the styliform element is the radius, instead. No evidence for this manner of preservation — longitudinal splitting of a long bone, without any fragmentation of the split margins, without any shrapnel of bone, and without considering that the elements I identified as fragments were in anatomical position already and thus consistent with the hypothesis my photoshopping is countermanding — elsewhere on the slab is used to support this, but it should be true [or I think it is] because I think my photoshopping technique — untested, untried, unrpoven outside my own belief that it works — is an accurate analytical tool. I prove that it’s not by using irregular and uncomforable layers of the slab (described by the authors as such) as if they were continuous regions of the bone-bearing layer and drew in “missing” material of the vertebrae, hips, and feet. I misunderstand how Occam’s razor works.”
Take a closer look and you’ll see the longitudinal splitting with fragmentation. I showed it at pterosaurheresies yesterday. Lot’s of people are on board with this. Be nice.
I’ve taken a look, and I frankly do not find any strong support for your position. It seems rather curious how you seem to be asserting a very particular split in the bone when no other portion of the skeleton is comparably damaged. All other material that suggests damage show it at the ends of long bones or breaks, but a longitudinal split is your only recourse to plopping the “styliform” structures into conventional identifications. The problem with this is that this is circumstantial AT BEST. It matters not that “lot’s [sic] of people are on board,” when those people are ignorant of the problems your use of pareidolia. This is how people still believe that images of Jesus or Mary in toast are REAL. I don’t think you should be crowing about them accepting your arguments.
And as I said, you misunderstand how Occam’s razor works. The razor is a form of logical fallacy called a fallacy of induction. It attempts to generalize that an observation held consistent through a small portion of a sample must be true to the whole of the sample, because otherwise would be more complicated. We are taught to reason inductively for the most part because we are THEN turned from that position to verification. We verify interatively, constantly, and in a reductive, not inductive, process. We eliminate potential results when they cannot be supported. But the fallacy of induction is always positive, always about proving a claim true, and not falsifying it. These are steps in the process.
Your identification of a split in the long bone also fails the test of parsimony by assuming that this one area of the slab has undergone a very unique and bizarre taphonomic process not observed elsewhere on the slab, amongst bones that are in consistent anatomical relation to one another, and in consistent taphonomic condition, where only ends are damaged, or mid-shaft portions broken. It ignored that when bones are observed on slabs that split lengthwise in a manner consistent with your argument it is between mainslab and counterslab, and not a feature of the element naturally. Assuming the long, falcate, and otherwise without jointed ended “styliform” bones are natural and consistent, present on one side in addition to the radius and ulna, preserved as they for nearly their entire length, is consistent. Unique, interesting, bizarre, but nonetheless consistent within itself. You detract from this in order to render these bones, with their different morphologies, “consistent” only with an idea that neomorphic bones don’t occur.
DPeters – I think people would be “nice” if you were scientific.
The “ulna splinter” hypothesis doesn’t stack up with the evidence.
The website pterosaurheresies (like your reptileevolution website) is pseudoscience. It’s a tragedy anyone reads it and/or takes it seriously.
That styliform thing is way too long to be the radius.